Organic and inorganic substances. Inorganic substances of the cell
For the first time, chemical substances were classified at the end of the 9th century by the Arab scientist Abu Bakr al-Razi. He, relying on the origin of the substances, divided them into three groups. In the first group, he allotted a place for mineral, in the second - for plant and in the third - for animal substances.
This classification was destined to exist for almost a millennium. Only in the 19th century, two of those groups were formed - organic and inorganic substances. Chemicals of both types are built thanks to the ninety elements included in the DI Mendeleev's table.
Group of inorganic substances
Among inorganic compounds, simple and complex substances are distinguished. The group of simple substances combines metals, non-metals and noble gases. Complex substances are represented by oxides, hydroxides, acids and salts. All inorganic substances can be built from any chemical elements.
Group of organic substances
The composition of all organic compounds without fail includes carbon and hydrogen (this is their fundamental difference from mineral substances). Substances formed by C and H are called hydrocarbons - the simplest organic compounds. The derivatives of hydrocarbons contain nitrogen and oxygen. They, in turn, are classified into oxygen- and nitrogen-containing compounds.
![]()
The group of oxygen-containing substances is represented by alcohols and ethers, aldehydes and ketones, carboxylic acids, fats, waxes and carbohydrates. Nitrogen-containing compounds include amines, amino acids, nitro compounds and proteins. For heterocyclic substances, the position is twofold - they, depending on the structure, can refer to both types of hydrocarbons.
Cell chemicals
The existence of cells is possible if they contain organic and inorganic substances. They die when they lack water, mineral salts. Cells die if they are severely depleted in nucleic acids, fats, carbohydrates, and proteins.
They are capable of normal life activity if they contain several thousand compounds of organic and inorganic nature, capable of entering into many different chemical reactions. Biochemical processes in the cell are the basis of its vital activity, normal development and functioning.
Chemical elements that saturate the cell
The cells of living systems contain groups of chemical elements. They are enriched with macro-, micro- and ultra-microelements.
- Macronutrients are primarily represented by carbon, hydrogen, oxygen and nitrogen. These inorganic substances of the cell form almost all of its organic compounds. And they also include vital elements. A cell is unable to live and develop without calcium, phosphorus, sulfur, potassium, chlorine, sodium, magnesium and iron.
- The group of trace elements is formed by zinc, chromium, cobalt and copper.
- Ultramicroelements are another group representing the most important inorganic substances of the cell. The group is formed by gold and silver, which has a bactericidal effect, mercury, which prevents the reabsorption of water that fills the renal tubules, which affects enzymes. It also includes platinum and cesium. A certain role in it is assigned to selenium, the deficiency of which leads to various types of cancer.
Water in the cell
The importance of water, a substance common on earth for cell life, is undeniable. Many organic and inorganic substances dissolve in it. Water is a fertile environment where an incredible number of chemical reactions take place. It is able to dissolve the products of decay and metabolism. Thanks to her, toxins and slags leave the cell.

This liquid is endowed with high thermal conductivity. This allows the heat to spread evenly throughout the tissues of the body. It has a significant heat capacity (the ability to absorb heat when its own temperature changes minimally). This ability does not allow sudden temperature changes to occur in the cell.
Water has an extremely high surface tension. Thanks to him, dissolved inorganic substances, like organic ones, easily move through the tissues. Many small organisms, using the feature of surface tension, stick to the water surface and glide freely over it.
The turgor of plant cells depends on water. It is water that copes with the support function in certain species of animals, and not any other inorganic substances. Biology has identified and studied animals with hydrostatic skeletons. These include representatives of echinoderms, round and annelids, jellyfish and anemones.
Cell saturation with water
Working cells are filled with water to 80% of their total volume. The liquid is in them in a free and bound form. Protein molecules bind tightly to bound water. They, surrounded by a water shell, are isolated from each other.
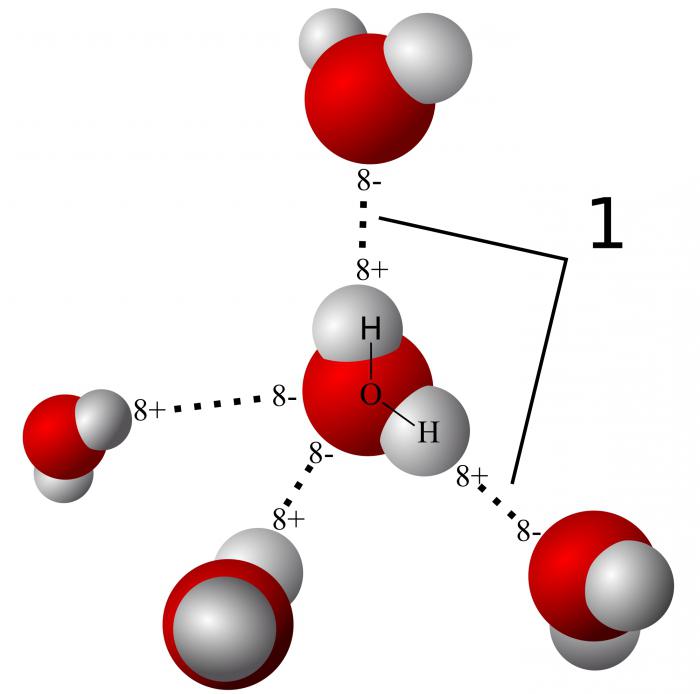
Water molecules are polar. They form hydrogen bonds. Due to hydrogen bridges, water has a high thermal conductivity. Bound water allows cells to withstand colder temperatures. Free water accounts for 95%. It promotes the dissolution of substances involved in cellular metabolism.
Highly active cells in brain tissue contain up to 85% water. Muscle cells are 70% saturated with water. Less active cells that form adipose tissue need 40% water. In living cells, it not only dissolves inorganic chemicals, it is a key participant in the hydrolysis of organic compounds. Under its influence, organic substances, splitting, are transformed into intermediate and final substances.
The importance of mineral salts for the cell
Mineral salts are presented in cells by cations of potassium, sodium, calcium, magnesium and anions HPO 4 2-, H 2 PO 4 -, Cl -, HCO 3 -. The correct proportions of anions and cations create the acidity necessary for cell life. In many cells, a weakly alkaline environment is maintained, which practically does not change and ensures their stable functioning.
The concentration of cations and anions in cells is different from their ratio in the intercellular space. The reason for this is active regulation aimed at the transportation of chemical compounds. This course of processes determines the constancy of chemical compositions in living cells. After cell death, the concentration of chemical compounds in the intercellular space and the cytoplasm is in equilibrium.
Inorganic substances in the chemical organization of the cell
In the chemical composition of living cells, there are no special elements characteristic only of them. This determines the unity of the chemical compositions of living and nonliving objects. Inorganic substances in the composition of the cell play a huge role.
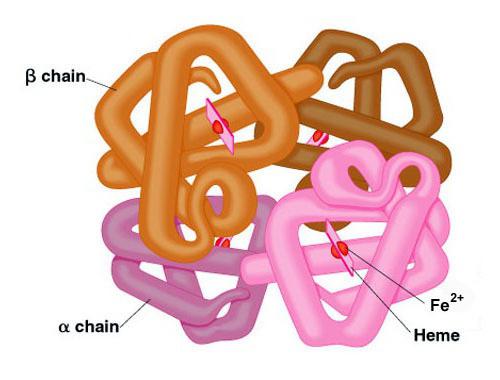
Sulfur and nitrogen help proteins form. Phosphorus is involved in the synthesis of DNA and RNA. Magnesium is an important component of chlorophyll enzymes and molecules. Copper is essential for oxidative enzymes. Iron is the center of the hemoglobin molecule, zinc is part of the hormones produced by the pancreas.
The importance of inorganic compounds for cells
Nitrogen compounds convert proteins, amino acids, DNA, RNA and ATP. In plant cells, ammonium ions and nitrates in the process of redox reactions are converted into NH 2, become participants in the synthesis of amino acids. Living organisms use amino acids to form their own proteins, which are necessary for building bodies. After the death of organisms, proteins are poured into the circulation of substances; during their decay, nitrogen is released in free form.
Inorganic substances, which contain potassium, play the role of a "pump". Thanks to the "potassium pump", substances that they urgently need penetrate into the cells through the membrane. Potassium compounds lead to the activation of the vital activity of cells, thanks to them excitations and impulses are carried out. The concentration of potassium ions in cells is very high, in contrast to the environment. Potassium ions after the death of living organisms easily pass into the natural environment.
Substances containing phosphorus contribute to the formation of membrane structures and tissues. In their presence, enzymes and nucleic acids are formed. Various soil layers are saturated with phosphorus salts to one degree or another. The root secretions of plants, dissolving phosphates, assimilate them. Following the death of organisms, the remains of phosphates undergo mineralization, turning into salts.
Inorganic substances containing calcium contribute to the formation of intercellular substance and crystals in plant cells. Calcium from them enters the blood, regulating the process of its coagulation. Thanks to it, bones, shells, calcareous skeletons, coral polyps in living organisms are formed. Cells contain calcium ions and crystals of calcium salts.
